Program Overview
Join the Sempulse Halo Pilot Program to Revolutionize Vital Signs Monitoring
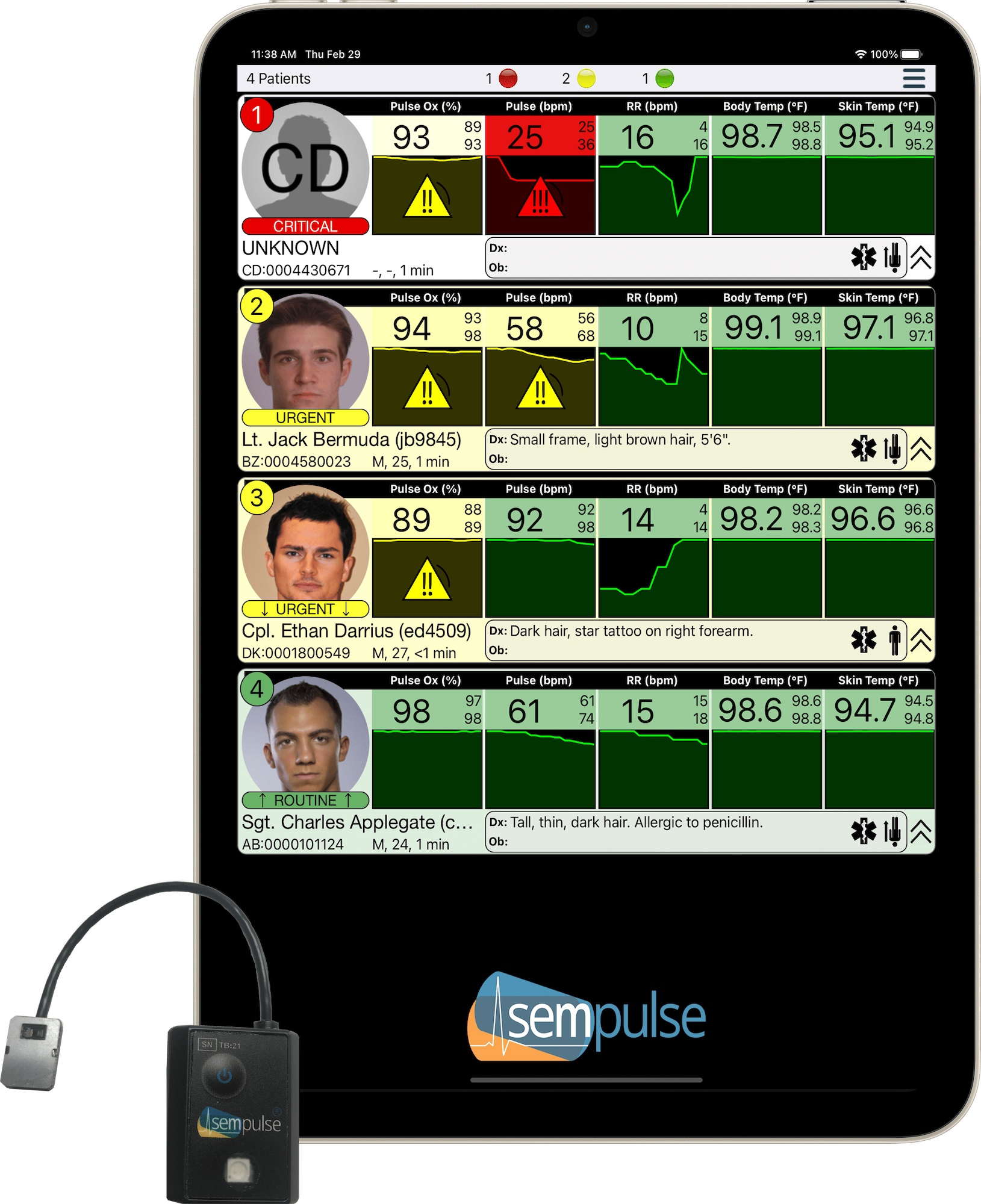
Sempulse is transforming emergency medicine with Halo, the world’s most advanced non-invasive vital signs monitor. Designed for military personnel, first responders, and healthcare providers, Halo delivers real-time, hospital-grade biometric data anywhere, without requiring invasive procedures.
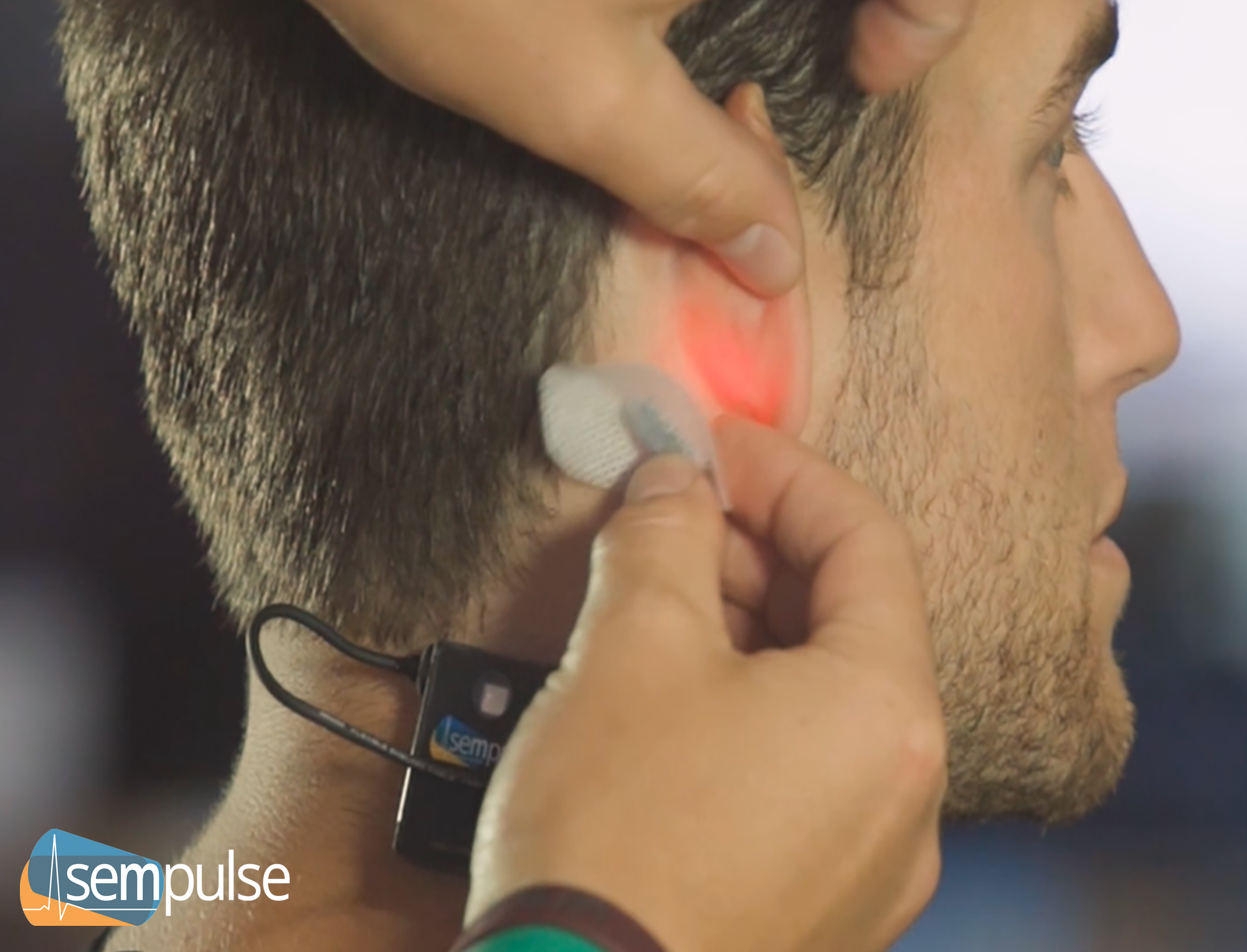
Sempulse’s FDA-cleared Halo monitor applies in seconds to the back of the ear and wirelessly monitors 100s of patients’ vital signs, non-invasively, during motion, and in austere environments. That’s pulse oximetry (SpO2), pulse rate, respiratory rate, core body temperature, and skin temperature within 45 seconds of application.
Our technology represents a capability leap for austere and extra-hospital patient monitoring. Because of this we have received a high volume of inquiries about trial and pilot programs. We wish we could support all of them, but we have to be judicious about where we spend our time and efforts in the short term. To that end we are requiring a modest paid pilot program as a means of qualifying those organizations interested in revolutionizing remote patient monitoring (RPM) in their organizations. All other organizations will be added to a waitlist that should make them eligible by the end of 2025.
Military, government, and charitable organizations can join the Sempulse Halo Pilot Program for free today.
Program Highlights
Paid Sempulse Halo Pilot Program participants receive:
- 2 Loaner Sempulse Halo Devices
- Sempulse SDK + System Documentation
- Program Kick-Off Online Meeting
- Online Halo Device Application Training Session
- Up to 5 Hours of Technical Support
Qualifications
Your organization must be able to accept FDA clearances for regulatory oversight.
If your organization provides a medical data aggregator service or interface, in order to qualify for the Sempulse Halo Pilot Program, your organization must have an in-production solution with at least 25 recurring clients.
All other organizations qualify for the program.
Application
Frequently Asked Questions (FAQ)
Sempulse In The Media

Sempulse® to Demonstrate and Showcase the Halo™ Vital Signs Monitoring System at the Operational Medicine Symposium 2025

Sempulse® Showcases Halo™ Platform Vital Signs Monitoring System at Austin4America Conference During SXSW 2025

Sempulse® Halo™ Platform Selected for Project Convergence Capstone 5 (PC-C5) with the U.S. Army
Sempulse® Positions FDA-Cleared Halo™ Platform as an Innovation Accelerator at PwC's 2025 Emerging Tech Exchange

Sempulse® announces U.S. FDA 510(k) Clearance and the Commercial Launch of the Halo™ Vital Signs Monitoring System